A Randomised Phase II Trial of Pre-operative cisplatin, 5 fluorouracil anddocetaxel ± Radiotherapy based on poor early response to standardchemotherapy for resectable adenocarcinoma of the oesophagus and/or OGJunction (10521)
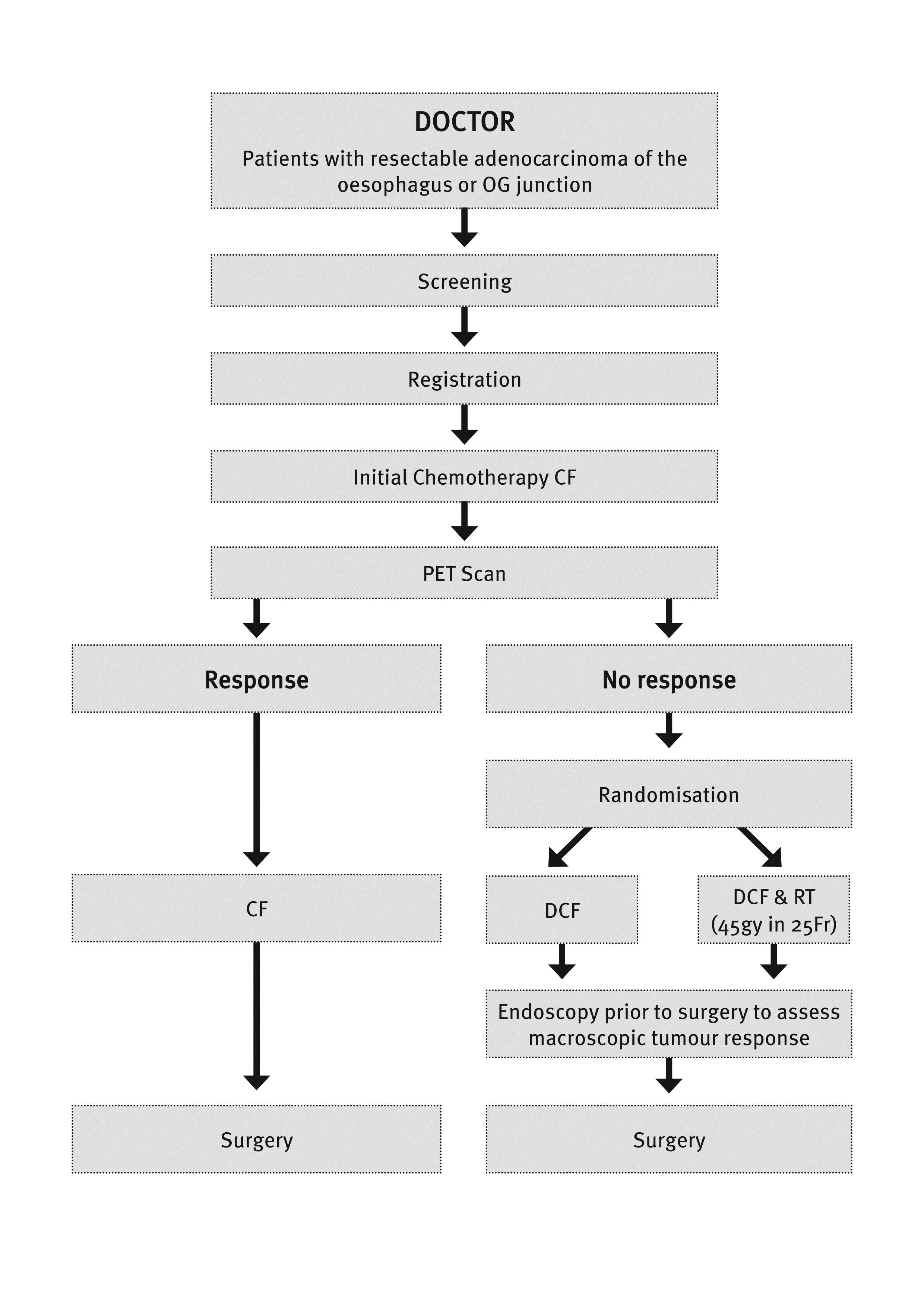
AIMS - This study represents a paradigm shift in the administration of pre-operative therapy for oesophageal adenocarcinoma (OAC). This will be the first study to focus on metabolic non-responders to pre-operative therapy in so far as the management of this group will be changed to a different therapy to try to improve response and potentially survival. This study will be the first to use the combination of DCF +/- RT in non-responding patients with OAC generating novel safety and efficacy data. It will determine whether changing therapy can salvage a response and provide valuable data regarding the potential to individualise therapy related to the tumour characteristics – so called ”tailored therapy”. Finally the routine pre-treatment tumour and blood banking will give our group a unique opportunity to search for OAC biomarkers that may be indicators for FDG-PET response, response to the regimens used and possibly assessment of survival. The primary aim is to assess whether changing the pre-operative therapy regimen to DCF +/- RT after the first cycle of chemotherapy for early metabolic non-responders to CF will induce a histological response. Study endpoints include: major histological response (<10% residual viable tumour) and secondary endpoints of PFS, OS, toxicity, HRQoL and feasibility of response-based therapy. In addition banking of tumour tissue and blood before and after treatment for future molecular analyses investigating biomarkers of response will be undertaken.
BACKGROUND – Over the last 30 years, the incidence of OAC in Australia has increased more than any other cancer. Surgery forms the mainstay of curative treatment, however survival remains poor. Preoperative chemotherapy (CTX) with or without concurrent radiotherapy (CRT) have resulted in modest improvements in outcome. Patients who demonstrate a histological response in the resected specimen following pre-operative therapy (CTX or CRT) have consistently better survival than non-responders. Recent data suggests that a reduction in the level of tumour activity seen on a PET scan performed 14 days after the start of CTX compared with a baseline PET scan (”early metabolic response”), is predictive of a histological response and improved survival. Increasing the proportion of responders to pre-operative therapy remains one of the major challenges facing patients with localised OAC. We believe it is time to undertake the first clinical trial aimed at improving outcomes for early metabolic non-responders by changing their therapy after the first cycle of standard CTX to include docetaxel +/- radiotherapy.
STUDY DESIGN - This is a randomized Phase II study for patients that are PET-non-responders to induction chemotherapy. Patients will be randomised equally to the two treatment groups. Additionally; information on a third group of patients who respond to induction chemotherapy will be collected to help inform a future Phase III trial.
A sample size of 30 patients per arm will have 80% power with 95% confidence to exclude an uninteresting histological response rate of 5% in favour of a 20% response rate in each arm. A formal comparison for response rates between the two randomised groups is not planned. This study will have 48 months accrual and 36 month follow up.
STUDY PROGRESS – The study was opened by Princess Alexandra Hospital in 2009, it was then awarded an NHMRC grant in 2010 which has enabled it to be a multi-centre trial coordinated by the AGITG/CTC. The study is now open at 9 sites - Princess Alexandra Hospital, Calvary Mater Newcastle, John Hunter Hospital, Townsville Hospital, Nepean Hospital, Royal North Shore Hospital, Royal Brisbane Women’s, Flinders Medical Centre and Royal Hobart Hospital. There are currently 65 patients registered (2 screen fails) and of those 30 are randomised. Interim analysis is currently underway. There is the possibility to open further sites across Australia.
TRANSLATIONAL RESEARCH - Tissue banking (normal and tumour) will be performed routinely for consented patients at two time points: (1) at pre-treatment endoscopy or EUS and (2) at surgery. These biopsy specimens are to be collected and stored as fresh tissue for any and all later molecular analyses. Further tissue will be obtained from the primary tumour in the resected specimen for tumour banking for any and all future molecular and genetic analyses. Tissue will be sent to a central laboratory for research. Blood will be obtained after informed consent at two time points: (1) at the time of routine pre-treatment blood tests and (2) at the routine pre-surgery blood tests. These samples will also be banked for long-term storage and future analyses. Serum and plasma for biomarkers will be collected and sent as fresh whole blood to the central lab for processing.
 AGITG 2013*
AGITG 2013*