Multicentre international study of capecitabine +/- bevacizumab as adjuvanttreatment of colorectal cancer (10524)
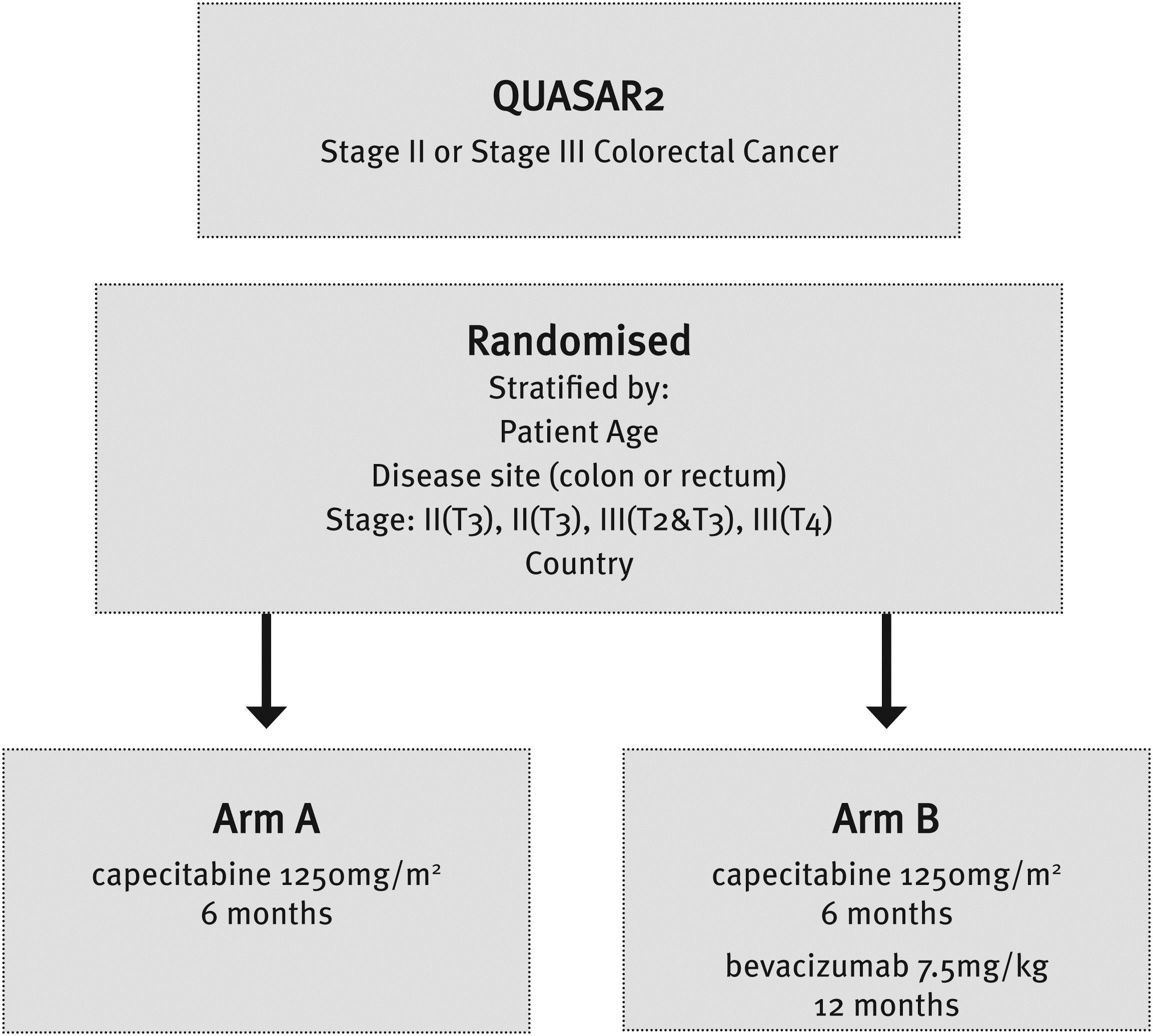
Aims - QUASAR2 tests the hypothesis that the combination of capecitabine (an oral chemotherapy agent) and bevacizumab (a molecularly targeted therapy against VEGF) is superior to capecitabine alone in preventing relapse in Stage II and III CRC.
This worldwide investigator driven phase III study, run by the OCTO group, recruited 2240 patients with stage III or high risk stage II CRC. Patients were randomised to capecitabine alone or capecitabine + bevacizumab. The primary endpoint of the study is disease free survival. Secondary endpoints include overall survival, side effect profile and translational science.
Study progress – QUASAR2 recruited patients in ANZ though the AGITG from July 2007 until September 2010. Initial allocation was 100 patients, which was later increased to 200 patients and then 250 due to rapid uptake. Final ANZ recruitment was 219/250 from 31 participating sites. Recruitment was above target (average 6 patients per month) until the allocation for Stage II was completed, then slowed.
Following an OCTO DSMC review of preliminary results from the AVANT study, the study was closed and AGITG complied with the recommendation that all QUASAR2 patients still on therapy cease treatment with bevacizumab. This was implemented at ANZ sites on 18th October 2010, at which time 25 patients were still enrolled (17 on the bevacizumab arm).
All QUASAR2 CRF submission will cease in October 2013. Data cleaning is expected to be completed during first quarter 2014, when final analysis will take place. A toxicity data poster was presented at ESMO 2012. A late breaking abstract may be submitted for ASCO 2014 depending on data cleaning timetables.
Translational Research – Consent to donate tissue for research was optional. Tissue collection is underway with 107 blocks of 163 being collected. Translational research studies are planned, with protocols being developed.
 AGITG 2013*
AGITG 2013* 