A Randomised Phase II Study Evaluating Potential Predictive Biomarkers in the Treatment of Locally Advanced and Metastatic Pancreatic Cancer (10538)
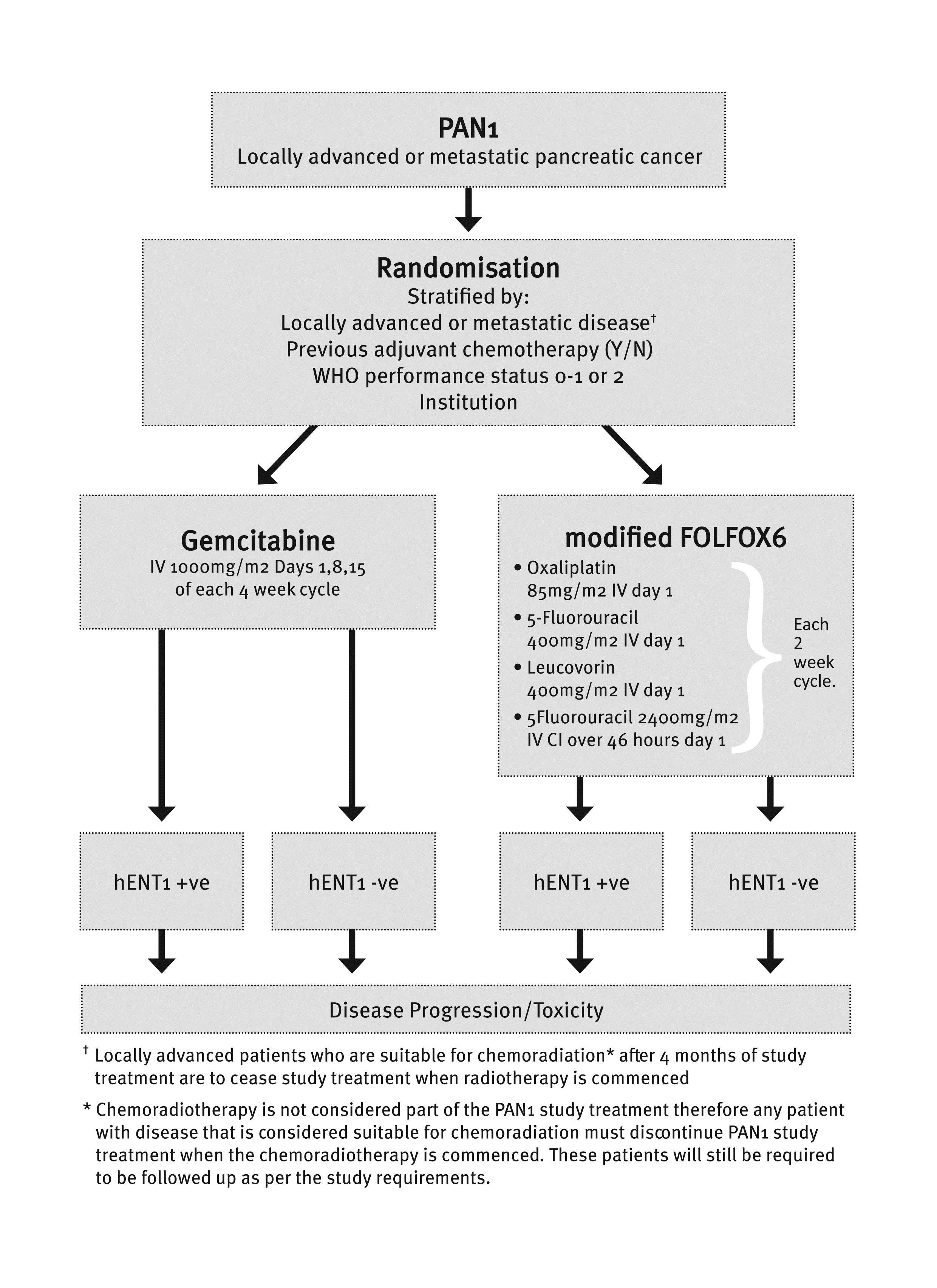
AIMS - The PAN1 trial aims to demonstrate the feasibility of hENT1 and biomarker directed treatment of pancreatic cancer. This randomised phase II exploratory study which will evaluate hENT1 as a predictive marker of benefit from gemcitabine treatment will inform the optimal design of future studies based upon this novel approach to the management of pancreatic cancer, and may be expanded to a phase III study if successful. In addition, it will also assess the efficacy/activity of gemcitabine and mFOLFOX6 in hENT1 positive and negative patients, the feasibility of biomarker assessment prior to treatment commencement, and the toxicity of gemcitabine and mFOLFOX6 in this population. The tissue which will be collected as part of the trial will include a cohort of gemcitabine and non-gemcitabine treated patients which will support novel predictive biomarker discovery.
BACKGROUND – Human equilibrative nucleoside transporter 1 (hENT1) is a ubiquitously distributed integral membrane protein which functions as a transporter of purine and pyrimidine nucleosides across the cell membrane, and hence appears to be an important mechanism for intracellular uptake of gemcitabine prior to its conversion to gemcitabine triphosphate, its therapeutically active form. Recent data from retrospective hENT1 analysis of existing pancreatic cancer patient cohorts suggests that hENT1 status may predict for a benefit from gemcitabine treatment.
Despite being the standard treatment for advanced pancreatic cancer for the past decade, the efficacy of gemcitabine in unselected patients appears to be modest. A multitude of randomised trials have tested the addition of other agents to gemcitabine and have largely failed to show improved survival with combination treatment based on gemcitabine. Although the exception, the addition of erlotinib, an oral tyrosine kinase inhibitor of the epidermal growth factor receptor, to gemcitabine resulted in only a small increment in median survival. These results suggest that gemcitabine may only be effective in some patients, and may not be the optimal platform for developing future combination systemic treatments for pancreatic cancer.
Further evidence supporting the use of non-gemcitabine containing combinations in the first line setting comes from a recently reported phase III trial (PRODIGE 4/ACCORD 11). In addition, the combination of oxaliplatin & infusional 5FU has been shown to be effective treatment after failure of gemcitabine. The use of a FOLFOX-type regime has advantages in that it is a regimen that is well established in colorectal cancer, and as such clinicians will be familiar with the logistics of treatment and managing associated toxicities. When compared to other infusional 5FU based schedules, such as FOLFOX4, the modified FOLFOX6 schedule is less onerous on patients as it requires less treatment time in clinic with fewer visits. This combination may therefore potentially be an appropriate alternative to gemcitabine in previously untreated patients, particularly in the hENT1 negative patients who might otherwise have less benefit from gemcitabine treatment.
STUDY DESIGN - This is a phase II, open label, randomised multi-centre trial. Patients will be randomised to receive either gemcitabine or mFOLFOX6 chemotherapy with stratification based on
• Locally advanced vs metastatic disease;
• Previous adjuvant chemotherapy (yes vs no);
• WHO performance status (0, 1 vs 2); and
• Institution.
Patients will continue their assigned treatment until progression, or unacceptable toxicity. Subsequent treatment will be allowed per clinician and patient preference.
Around 80 patients will be randomised to the study, 40 hENT1 positive patients (20 receiving gemcitabine and 20 receiving mFOLFOX6) and 40 hENT1 negative patients (20 receiving gemcitabine and 20 receiving mFOLFOX6).
STUDY PROGRESS - The protocol was initially approved by the NSW Cancer Institute for Central Human Research Ethics committee on 25th January 2011 and the first site activated in July-2011. The trial was funded by the Avner Nahmani pancreatic cancer research fund.
The protocol was amended (approved June-2012) to allow locally advanced pancreatic cancer patients onto the PAN1 study. The timeline for the hENT1 result was also revised to allow patients to be randomised onto the study prior to the result, as long as the tissue for testing is available. hENT1 will still be assessed prospectively, but this will occur in batches during the study and the results will be available before the patient outcomes are known, so that the effect of hENT1 can still be evaluated. These changes do represent significant modifications to the study design, but are important to preserve the overall aim of the trial, which remains to evaluate hENT1 and other potential predictive biomarkers in the treatment of pancreatic cancer.
The PAN1 study Trial Management Committee recommended closure of the study to further randomisation, effective from Friday the 22nd of February 2013 due to poor recruitment. In addition, the Trial Management Committee were concerned that the relevance of the treatment arms in this phase 2 study may have been surpassed by recently reported data from randomized trials which have demonstrated an improved outcome from the addition of nab-paclitaxel to gemcitabine such that there may be increasingly fewer patients for whom gemcitabine monotherapy remains suitable treatment. This was reported to the CINSW HREC on the 13th March 2103. At the time recruitment ceased there were 13 sites activated and 16 patients recruited to the trial.
Although PAN1 has closed to further randomisation, patients who are on study will continue with their assigned treatment, and be followed up as per study protocol. There have been no safety concerns related to the trial and the decision to close recruitment does not relate to any interim results from the trial. The CTC will work closely with those sites that do not have any patients on the PAN1 study to expedite closeout of the study.
TRANSLATIONAL RESEARCH - There are two separate aspects to sample collections for PAN1:
- hENT1 testing sample
- Biomarker research samples
Biological samples from patients will be used for research purposed to identify biomarkers that are prognostic from pancreatic cancer or predictive of treatment response and that my help to better understand the pathogenesis, course and outcome of pancreatic cancer and related diseases. Peripheral blood and tissue samples will be collected from trial patients for this biomarker research
Serum, plasma and the red cell pellet will be isolated at sites at each time point according to the protocol. Frozen samples will then be sent to a central lab for storage and research. Biomarker data will be analysed to determine associations between their levels at baseline, and/or changes during treatment, and clinical outcomes including objective tumour response, progression-free survival and overall survival.
All samples for the hENT1 testing have been sent to the Canadian lab and we are waiting for the results. The remaining biomarker samples are stored at site and will be requested at the end of the study.
 AGITG 2013*
AGITG 2013*